Allergen immunotherapy
More information for you
Allergen immunotherapy (AIT), which used to be known as hypo-sensitization or desensitization, is the only therapy option that treats the root cause of IgE-mediated allergic disease.
As a disease-modulating treatment, it is effective and well-tolerated by patients with allergic rhinitis, allergic asthma and insect venom allergies. The most common approach is to gradually increase the dose of the allergen extract to modify the immunological response to the allergen.
This method activates specific blocking antibodies, tolerance-inducing cells and neurotransmitters, preventing further amplification of the allergen-induced immune response, blocking the specific immune response and suppressing the inflammatory response in tissues.
-
Immunotherapy vs. symptomatic treatment
A study based on meta-analyses that compared the effectiveness of subcutaneous immunotherapy (SCIT) with pharmacotherapy, revealed that there is “indirect but consistent evidence that SCIT is at least as potent as pharmacotherapy in controlling the symptoms of SAR as early as the first season of treatment”.
The study reviewed meta-analyses with five or more randomized, double-blind, placebo-controlled trials of SCIT, analyzing the nasal corticosteroid mometasone furoate, the leukotriene receptor antagonist montelukast and the antihistamine desloratadine (figure: Matricardi PM et al. J Allergy Clin Immunol 2011)
-
Reasons that speak for AIT
AIT is effective
- against IgE-mediated allergic rhinitis, rhino-conjunctivitis with or without allergic asthma, both in children and adults. Randomized, double-blind, placebo-controlled studies have shown that the need for symptomatic medication for nose, eyes and lung symptoms can be reduced.
- against controlled allergic asthma (GINA 2007) or intermittent and mild persistent asthma (GINA 2005). It reduces the need for inhalational corticosteroid while maintaining asthma control. In response to recent studies, AIT has been included as a therapy option in the latest German S2k guidelines on asthma management, and in the 2018 German National Disease Management Guideline for Asthma in the medicinal step-by-step plan for adults, children and adolescents, for any indications that justify this approach.
It has
- lasting effects, meaning that the benefits of AIT can continue to be felt long after the treatment ends
- preventive effects, as it protects against progression, e.g. the development of asthma in patients with allergic rhinitis
- preventive effects, as it can impede the development of new sensitizations
It can
- improve the quality of life of patients with allergic rhinoconjunctivitis
It is
- “safe and well tolerated, if it is applied correctly, if patient selection is based on the indication, and if it is performed in a practice/clinic experienced with this therapy” (AIT Guidelines, Pfaar et. al 2022)
- more cost-effective than symptomatic therapy for allergic rhinitis and allergic asthma in the long term
-
Indication
AIT is indicated for:
- patients with moderate to severe intermittent or persistent allergic rhinitis/rhino-conjunctivitis and/or at least partially controlled allergic asthma; also, patients with 2) and 3) below, mild symptoms, whose treatment goal is a disease-modifying effect
- corresponding clinically relevant sensitization
- symptoms persisting despite symptomatic therapy and/or avoidance measures
proof of effectiveness for indication and age group
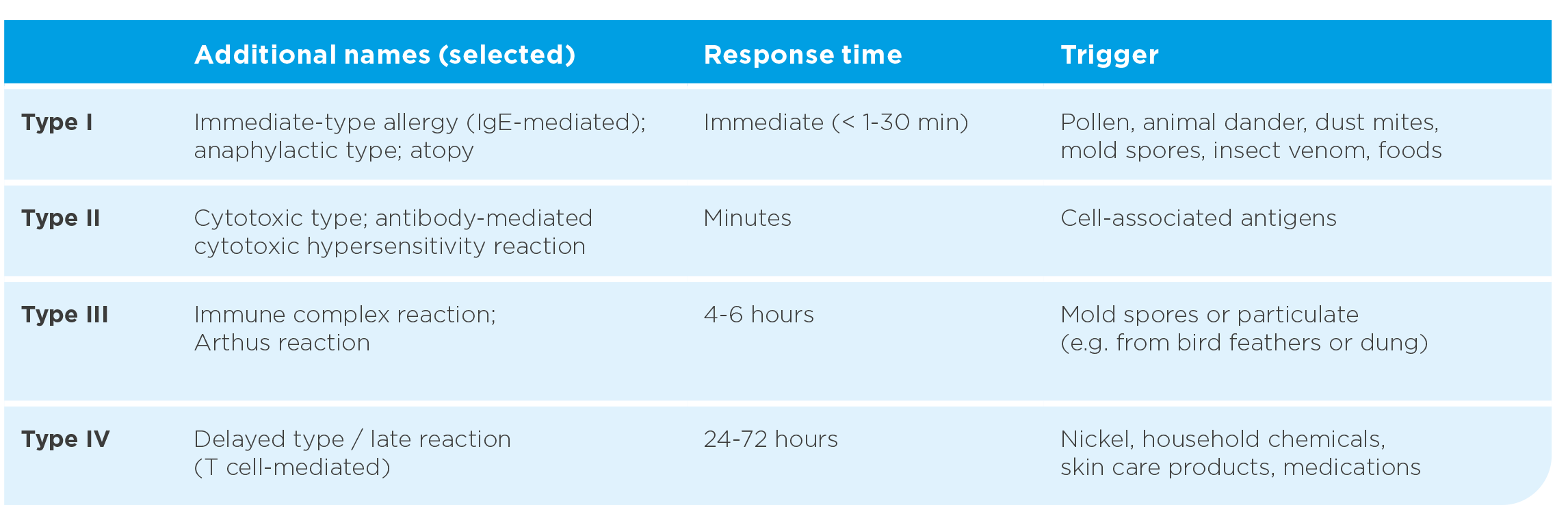
Table: Classification of allergic rhinitis according to ARIA (Allergic Rhinitis and its Impact on Asthma). (mod. n. Bousquet J et al. J Allergy Clin Immunol 2001; Scadding GK et al. Clin Exp Allergy 2017)
-
Boosting effectiveness
There are a number of parameters that impact the effectiveness of AIT with inhalational allergens, such as
- optimal dosage
- high cumulative dose
- allergen extract quality
- preparations that contain individual allergen types or a mixture from a homologous group (of the same botanical family) (in patients with allergies against grass pollen, this would be tree pollen or house dust mites)
- good compliance and adherence
Patient compliance and adherence are important, as for maximum effectiveness and a lasting effect, patients should receive AIT for at least three years. Discontinuing AIT before completing three years of treatment and/or deviating from the manufacturer’s recommendations on how to administer allergen extracts can drastically diminish treatment effects. Patient adherence appears to be lower with SLIT (sublingual immunotherapy) than with SCIT.
-
Tolerance
Redness and/or local reactions at the SCIT injection site are common. These side effects may be managed with cold compresses, topical corticosteroids or systemic antihistamines. Systemic side effects are less frequent. They can be mild to severe and affect the skin, the gastrointestinal tract, the upper and lower respiratory tract or the cardiovascular system. They are easily treated.
Potentially life-threatening systemic reactions caused by SCIT are extremely rare as long as all safety precautions are observed (see list of risk factors below). Between 1991 and 2000, the Paul Ehrlich Institute (PEI) reported a relative frequency of serious systemic reactions following 0.0005 % to 0.01 % of injections with modified semi-depot preparations (allergoids), and following 0.002 % to 0.0076 % with unmodified semi-depot preparations. An analysis of these reports of serious systemic reactions showed that approximately 37 % could have been prevented.
If serious side effects occur during SCIT, switching to SLIT is not recommended. In these cases, SLIT has the potential of causing a serious systemic reaction.
Premedication with antihistamines may reduce the extent of local reactions in both SCIT and SLIT, but does not reduce the risk of a systemic reaction.
Serious systemic reactions to AIT can, in part, be explained by risk factors, such as
- high degree of sensitization/ sensitization to animal allergens or pollen
- uncontrolled, inadequately treated asthma
- a previous anaphylactic reaction during AIT
- current allergic symptoms/ allergen exposure (including after discontinuation of symptomatic medication)
- acute infection, mast cell disease or elevated tryptase level
- use of β-blockers
- physical stress (e.g. physical exertion, sauna bathing, excessive alcohol consumption, extreme cardiovascular stress)
- inadequate dose increase or allergen extract overdose
- SCIT only: inappropriate injection method, e.g. accidental intravascular injection
-
Administration of SCIT
In practice
Practices/clinics administering AIT should adhere to the following recommendations:
Physicians
- experience with diagnosis and differential diagnosis of allergic rhinitis
- (additional) training in allergology or sufficient experience with AIT
- ability to identify and treat severe systemic reactions, including anaphylaxis
Staff
- trained in treating severe allergic reactions
Practice
- observation room for patients after receiving AIT (30 minutes minimum)
- first aid equipment
Before starting AIT, patients/parents of pediatric patients must be informed about the
- alternatives to AIT
- chance of success
- recommended treatment duration of at least three years (adherence and persistence)
- possible side effects
- procedure in the practice (appointments, observation time after injection)
- qualification and accessibility of the practice
- behavioral rules
The patient should be educated about AIT and provided with a written patient information sheet(“Therapy information sheet“). We recommend that patients sign a written agreement. Patient education and consultation should be documented.
It is recommended that patients receive AIT for at least three years, as its effectiveness depends on the cumulative allergen dose. If symptoms persist or if the dose has been reduced, the therapy may be extended. For patients who show no response to the therapy (“non-responders”) after one or a maximum of two years, the diagnosis and indication should be critically reviewed. In some cases, it may be appropriate to change the preparation or to switch from a pre-seasonal pollen AIT to a perennial therapy. Discontinuation is also an option.
“Hyposensitization vaccines for injection may only be prescribed and administered by physicians trained or experienced in allergy” (Paul Ehrlich Institute, communication dated April 5, 1995)
-
Mechanism of action
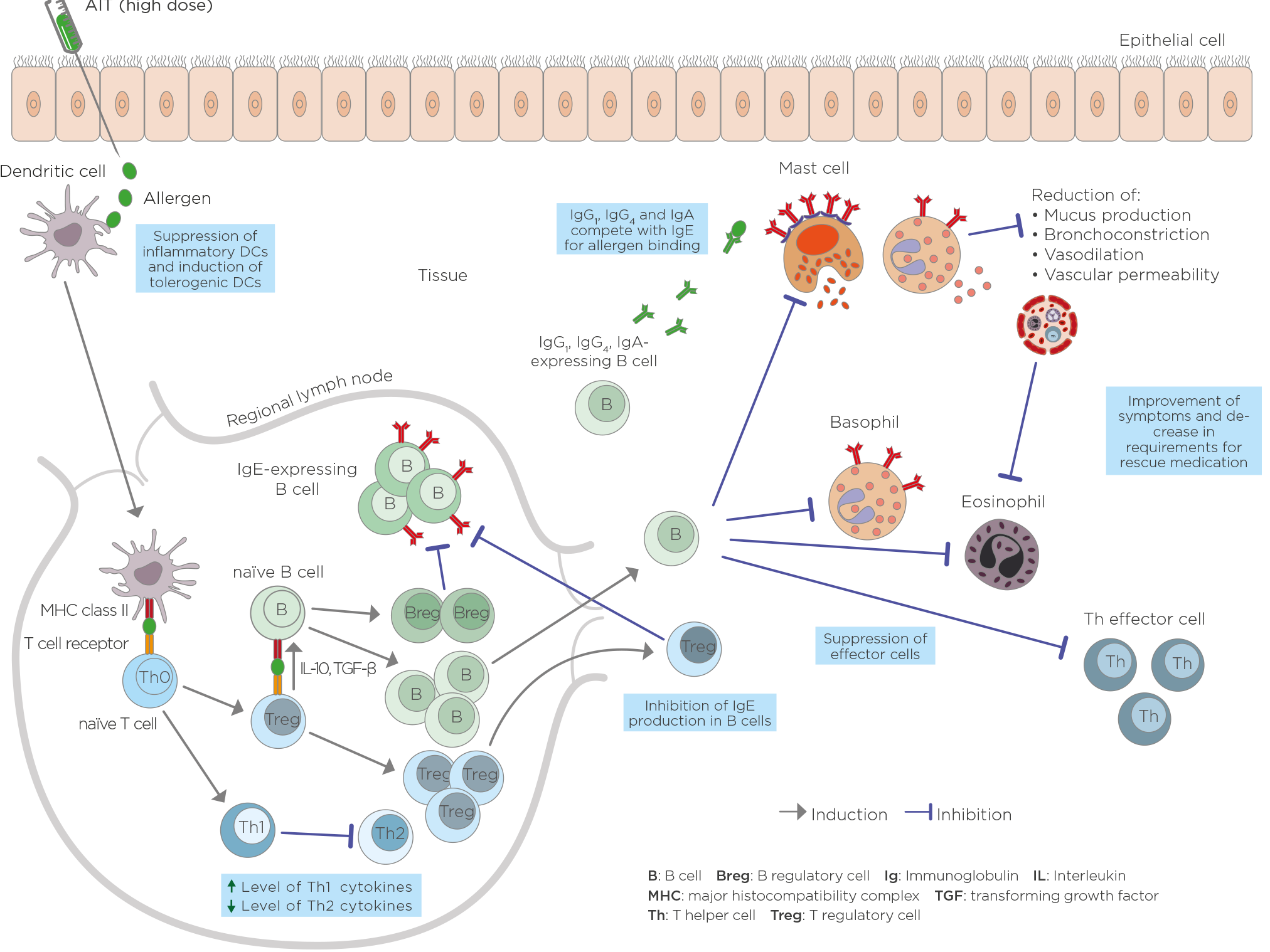
Mechanisms of AIT at a cellular level (based on Palomares O et al. Immunol Rev 2017; Shamji MH, Durham SR. J Allergy Clin Immunol 2017; Fujita H, et al. Clin Transl Allergy 2012; Larché M, et al. Nat Rev Immunol 2006; 6(10):761–71)
The mechanism of action of AIT is diverse and complex and involves various processes. However, the details of the mechanism are still not fully understood.
Dendritic cells turn allergens into fragments (peptides), which form a complex with MHC class II molecules. This complex is presented on the surface of dendritic cells and recognized by the t-cell receptors of naïve t-cells. Unlike natural allergens, which induce a differentiation of t-cells into Th2-cells in allergy-prone patients, the high allergen dose administered in AIT adjusts the dendritic cells’ function, shifting the immune system towards a more balanced, or a Th1, response. This results in a decrease in Th2-cytokines such as IL-4, IL-5, IL-9 and IL-13. Another important mechanism in AIT is the generation of allergen-specific regulatory t-cells (Tregs) that produce anti-inflammatory cytokines, such as IL-10 and TGF-ß, as well as regulatory B cells (Bregs). Tregs inhibit the production of allergen-specific IgE and induce IgG4 production in B cells. B cells additionally promote the production of IgG1, IgG4 and IgA antibodies.
Allergen-specific IgG, especially IgG4, competes with IgE for binding allergens because it recognizes the same epitope. This prevents high-affinity IgE receptors from cross-linking to basophils and mast cells, reducing degranulation and the production of histamines. The resulting decrease in mucus production, bronchoconstriction and vascular permeability reduces allergy symptoms. The inhibition of vascular permeability leads to reduced effector cell recruitment to the tissue affected by the allergic reaction, thereby reducing the inflammation of that tissue. Treg cells also minimize the allergic response by suppressing mast cells, basophiles, eosinophils and T-effector cells. In addition, Treg cells can interact with resident tissue cells and help remodel the tissue.
The immunological changes during AIT have been shown to follow a specific chronology. Typically, the first injection leads to degranulation of mast cells and basophils and a decreased tendency for systemic anaphylaxis. Then allergen-specific Treg cells are generated while the production of Th2 cells, and probably also effector cells, is suppressed. Specific IgE shows an early increase and decreases relatively late. Especially the IgG4 antibody level rises early, depending on the applied dose. Numerous studies have also shown a rise in allergen-specific IgG1 and IgA levels. After several months, the allergen-specific IgE/IgG4 ratio decreases. A significant decrease in type I skin test reactivity is also observed relatively late in the course. After a few months, decreases in tissue mast cells and eosinophils and release of their mediators and skin late phase response occur.
The clinical efficacy, immunological response and safety of AIT are subject to a dose-response relationship.